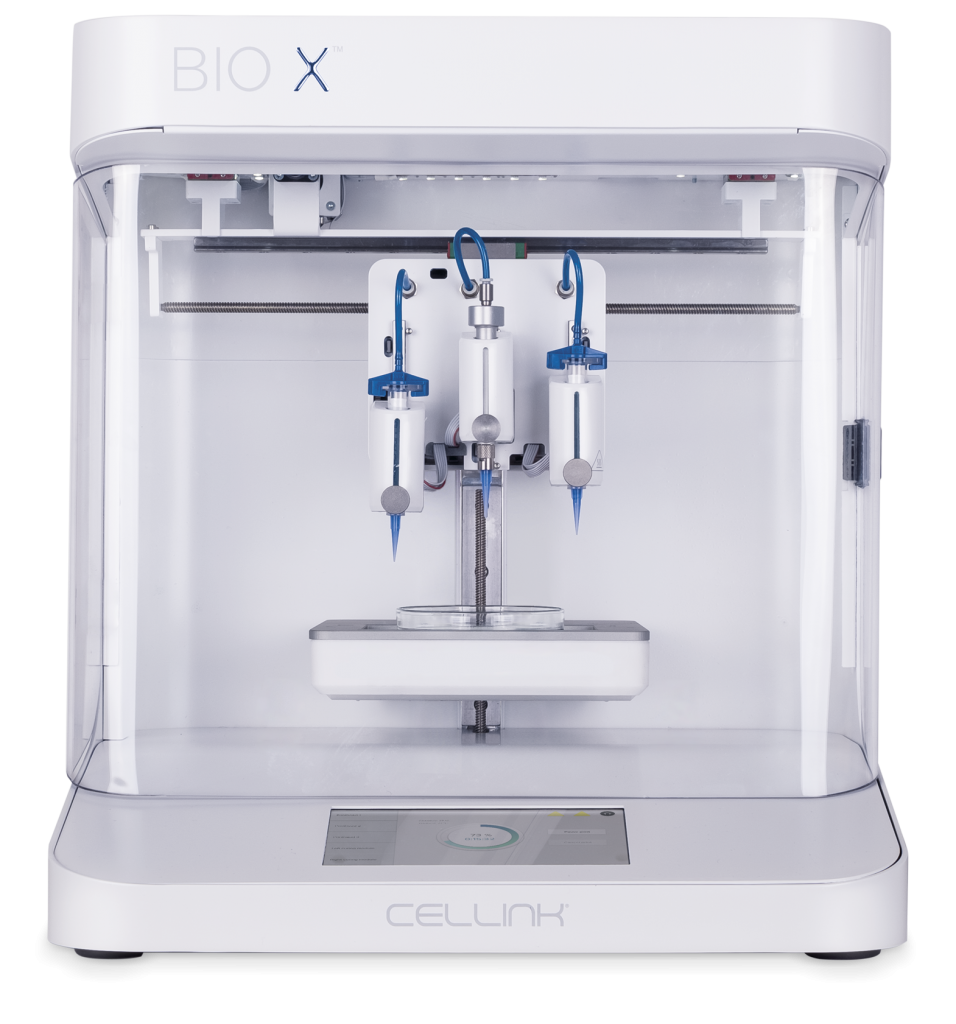
Our Products
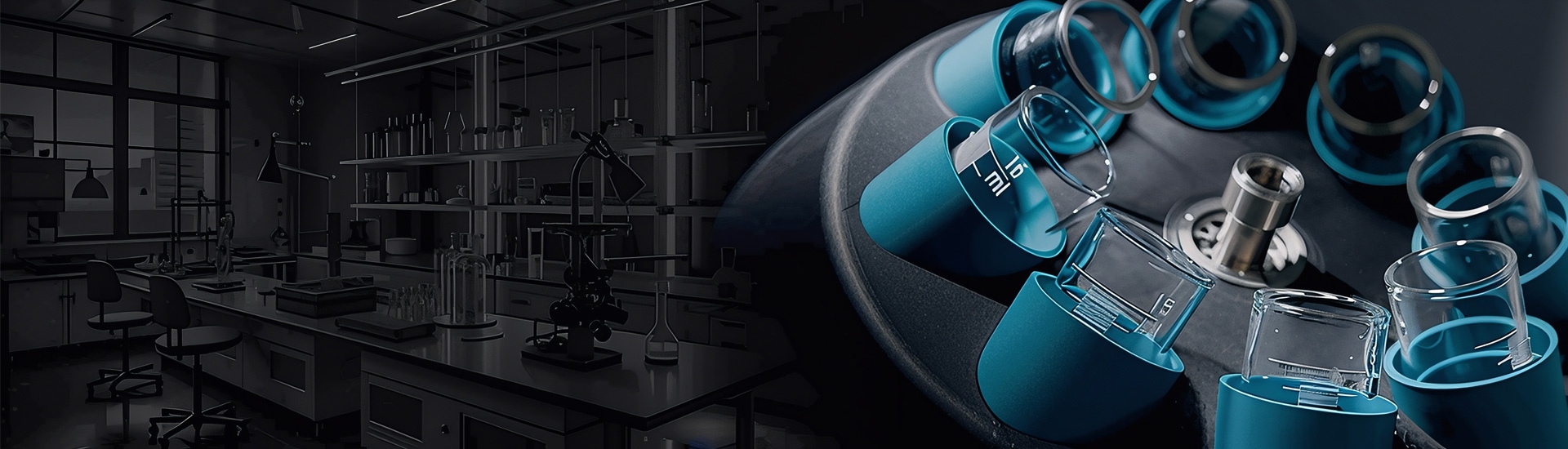
Our expert team brings extensive experience in the scientific field, delivering high-quality laboratory and scientific equipment. We proudly serve universities, research organizations, hospitals and industries with reliable solutions that support innovation, precision, and progress.
Welcome To
Gaia Science
At Gaia Science Pte Ltd, we take pride in being an all-in-one solution provider for the scientific community. With a professional team possessing a collective wealth of experience in the scientific industry, we deliver end-to-end solutions — from equipment supply and laboratory consultancy to design, renovation, and turnkey project execution.
We supply a comprehensive range of scientific and laboratory equipment to universities, research institutions, healthcare facilities, and industrial sectors across the region. Recognized for our ability to bring cutting-edge technologies from our global partners and principals to our valued customers, we bridge the gap between innovation and application.
Our products and solutions are widely used in various disciplines, including genomics, proteomics, cell biology, animal research, organic synthesis, pathology, imaging, materials science, nanotechnology, advanced manufacturing, clean energy research, environmental analysis, education and many more.
Beyond supplying laboratory instruments, Gaia Science provides Turnkey Laboratory Solutions that include laboratory consultancy, planning, design, renovation, and equipment installation.
As an integrated laboratory solutions partner, our mission is to bridge state-of-the-art technologies to the scientific community, and our goal is to deliver service excellence. We see the scientific community as our partner in advancing science and technology for a better world and improved quality of life.

For any queries or support, kindly get in touch with us.
Ongoing Promotions
Enjoy exclusive deals and special savings happening right now for a limited time.
Featured Products
We're Here to Help
Fill out the form below and our team will get back to you shortly with the support you need.