Neurons are the targets of injury and disease in many neurological conditions, and achieving neuronal survival/repair is a key goal for regenerative medicine. In this context, genetic engineering of neurons offers a platform for (i) basic research to enhance our understanding of neuronal biology in normal, disease/and injury conditions; and (ii) for regenerative medicine to enhance the functionality of neurons. Although, a wide range of attempts have been made to promote gene delivery to primary neurons, these cells are still difficult to genetically engineer, and current methods rely heavily on viral vectors which pose safety considerations. Magnetic nanoparticles (IONPs) are currently of great interest in regenerative medicine including for non-viral gene delivery by the 'magnetofection' strategy, i.e when used with applied magnetic fields. This project aimed to examine (i) the influence of two novel uniaxial and biaxial oscillating magnetic field devices on primary neuronal transfection efficiency, and (ii) examine the safety of magnetofection using histological and electrophysiological studies. In order to do this, a robust protocol to derive primary cortical neurons was first established.
A second issue is that surgical delivery of Neurons results in low survival. Additionally, most basic research has relied on neurons grown on 'hard‘ substrates such as plastic, which do not mimic the mechanical properties of the in vivo microenvironment. To address these limitations, primary cortical neurons were grown in a 3-dimensional 'soft' collagen hydrogel construct which can serve both as a protective cell delivery system and a 'neuromimetic' substrate. The safety of the established protocol was evaluated by electrophysiological analyses on neurons.
The findings demonstrate that the safety of magnetofection is magnetic field dependent, and at optimal conditions, electrophysiological properties of the nano-engineered neurons were normal. Secondly, I have shown that collagen hydrogels can support the 3D growth of neurons and electrophysiological studies can be carried out on the construct neurons; small differences were found between neurons grown on hard and soft materials. Finally, the amenability of genetic engineering of neurons within hydrogels using IONPs has been shown.

 SingaporeSG
SingaporeSG ChinaCN
ChinaCN MalaysiaMY
MalaysiaMY IndonesiaID
IndonesiaID MyanmarMM
MyanmarMM_pddqnqv8.png)


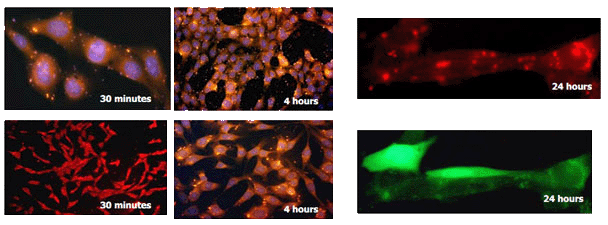
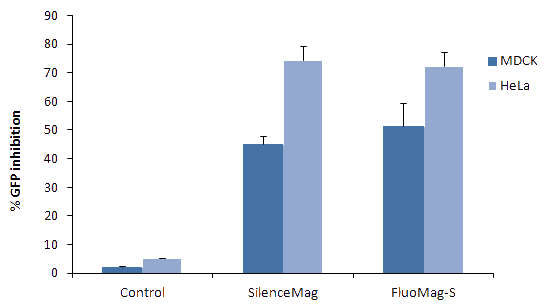
_fduw5yw6.jpg)
_7kqejgw9.jpg)
_eqh94p5i.jpg)
_es2etcej.jpg)